Food & Drug Administration
Design Date
November 2025
Challenge
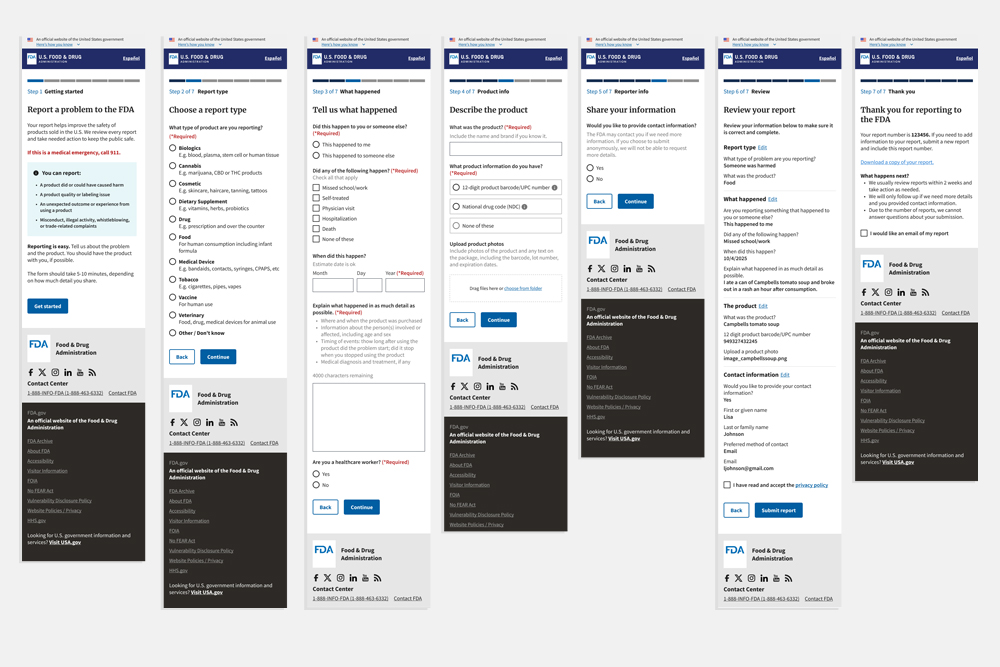
The FDA receives over 250,000 product-related complaints each year, but existing submission channels MedWatch Online, SRP, VAERS, email, mail, and fax, were outdated, fragmented, and frustrating for users. This made it difficult for the public to submit accurate reports and for the FDA to quickly act on potential safety issues. The challenge was to retire multiple legacy systems and create a single, easy-to-use experience that users could complete in under 10 minutes.
Solution
A unified complaint submission platform was designed to address major usability barriers and simplify the reporting process. Through research, iterative prototyping, and close collaboration with FDA stakeholders, the team streamlined the form flow, clarified content, and applied U.S. Web Design System components and FDA style guidelines to ensure clarity, accessibility, and trust.
Result
Over the course of five months, we conducted four rounds of usability testing with a total of 30 participants. Insights from each round informed iterative improvements to content hierarchy, form structure, and design. The final result was a clear, easy-to-use form that enabled successful submissions and actionable complaint data for the FDA post-market surveillance and consumer safety efforts.